Key recommendations for beverage quality control teams
The measurement of pH is often a critical quality parameter for a range of beverages, and most produced beverages will have a pH specification at every criticalcontrol point throughout their manufacture. However, pH electrochemistry is often poorly understood by Quality Control (QC) Managers and QC teams. This often leads to unnecessary inefficiencies caused by delays in obtaining readings, inaccurate measurements being recorded between different staff members and even the wrong process decisions being made.
This knowledge gap is being compounded by two clear trends facing QC teams in today’s modern beverage production environment. Firstly, retailers are demanding increased quality standards from their suppliers which is driving a need for greater control of laboratory procedures and consistency between QC team members. Secondly, in the drive for quicker results, there is a growing trend for measuring and recording critical pH measurements in the production environment, as opposed to the lab. Typically, this means personnel responsible for production, who may not have a science background, are responsible for obtaining and logging pH measurements—even though the data is still the responsibility of the QC team.
Given these trends, it is becoming increasingly important that QC Managers and members of the QC team have a greater technical understanding of pH measurement processes and principles. This will not only help the immediate QC team to obtain and record more consistent and accurate results, but it will also help when training other staff members to ensure they are signed-off as competent when taking critical pH measurements.
This Smart Note has been developed to provide a deeper understanding of pH measurement equipment, which in turn will assist with SOP development, staff training and ultimately improve the efficiency of laboratory operations.
How electrodes work
The term pH is derived from a combination of “p” for the word “power” and “H” for the symbol of the element Hydrogen. Together the meaning is the hydrogen ion exponent, where pH is the negative logarithm of the hydrogen ion activity. Sometimes this is referred to as the power of hydrogen.
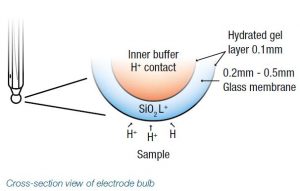
For a pH electrode to tell you how acid or alkali your sample is and relate this to a pH value on your meter, it needs to sense the level of hydrogen ions within your sample, then relay this information to the attached meter. To do this, it relies on knowing the changes in the relative hydrogen ion concentration inside and outside of the glass bulb on the electrode. These changes are facilitated by that bulb being made of a very specific glass ionic lattice that becomes hydrated in water samples, turning the outer and inner layers of glass into a gel.
The hydrated gel layers allow an ion exchange reaction in which hydrogen ions persist, which creates a difference in concentration between the outer surface of the bulb (in contact with the sample) and the inner surface of the bulb (in contact with a constant pH solution). The difference in hydrogen ion concentration across the glass bulb is registered as a mV signal which is transferred to the meter to express a pH measurement.
If you always store and maintain your electrode properly and keep the glass bulb wet, then you can spend less time calibrating and testing, and more time doing the important jobs in the lab!
The role od calibration standards and buffers
Regular calibration of the electrode and meter system as a whole is necessary as differences can exist between electrodes due to varying conditions such as; age, usage, and storage. These differences lead to changes that can affect the accuracy of the reading.
Calibration is conducted using specially prepared salt solutions with stable and defined pH values. These solutions are known as “standards” or sometimes “buffers”. The on-going accuracy of the electrode and meter system is therefore dependent upon the accuracy and stability of the calibration standard and the methods of calibration.
At Thermo Fisher Scientific, we use calibration standards that are directly traceable and fully documented to the National Institute of Standards and Technology (NIST) – the highest level available for pH calibration standards. This ensures the on-going accuracy and reliability of the measuring systems.
The requirement for electronic data records
The measurement and recording of accurate and consistent quality data from batch-to-batch is becoming increasingly expected by retail customers who wish to see this during quality audits. Whether for maintaining accurate sample testing reports for traceability purposes, or to demonstrate conformity to a compliance or regulatory standard, being able to retrieve accurate data records is now a key requirement.
While some laboratories still rely on manual entry of data into notebooks or similar logging methods, most modern meters now facilitate saving data to the meter’s internal log, allowing operators to back-up their sample measurement data along with calibration records to the meter’s non-volatile memory.
Stored data can then be exported from the meter to a printer, or computer, for additional long-term data retention. By automating tedious manual data logging, Quality Control (QC) Managers and QC teams can improve productivity, back-up critical data on multiple systems and share data more easily with colleagues, customers and auditors rapidly in digital formats.




