What are Medical Masks?
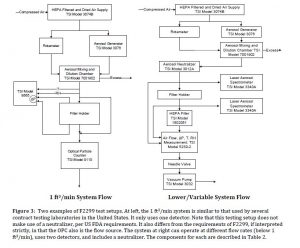
Medical masks are designed to be worn by health professionals during healthcare procedures. They are used with the intent of protecting both the wearer and the patient from infections spread by inhalable droplets and aerosols; see Figure 1. The category of “medical masks” includes procedure masks (also called “isolation masks”) and surgical masks.
A distinct but closely-related category of medical protection devices is surgical respirators. Surgical respirators – also called medical respirators – are a type of respirator designed for use in medical settings. While surgical respirators do not fall into th “medical mask” family, they are also required to be tested to ASTM F2100 standard.
Surgical masks are typically worn by operating room personnel during surgeries and frequently have ties. Surgical respirators are similar to surgiacl masks but also meet the more stringent filtration performance requirements of a respirator. Procedure masks may have elastic ear loops and are often used for medical procedures outside of the operating theater.
The COVID-19 global pandemic has resulted in a more widespread use of these masks. Consequently, requirements for quality control and performance verification of these masks have increased as well.
How are Medical Masks Certified?
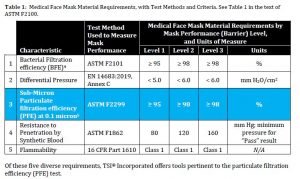
Medical mask certification around the world is governed by national and international standards. ASTM F2100 is one of the more widely-used international standards and hence is the focus of this document. This standard requires the material used to construct medical face masks to demonstrate certain levels of performance in five key characteristics: bacterial filtration, breathability (differential pressure), particulate filtration, blood resistance, and flammability.
Rather than providing detailed test methods for each of these areas, ASTM F2100 is a “parent” method that calls upon several other published methods. Results form tests according to those five published methods can result in a medical mask falling into one of three categories. See Table 1 for more details.
TSI Tools for Measuring the Submicron Particulate Filtration Efficiency (PFE) of Medical Masks and Respirators
PFE Testing According to ASTM F2299: General Requirements
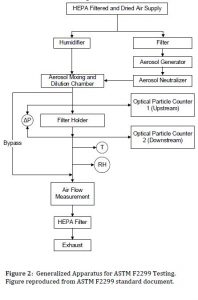
Test Method ASTM F2299 is relied upon by ASTM F2100 for determining the submicron particulate filtration capabilities of medical mask media. F2299 is a general method designed for different uses, and it allows a wide range of potential sizes of challenge particles, 0.1 to 5 μm. Compliance with F2100 can be achieved when using nominally 0.1 μm polystyrene latex spheres (PSL) as the challenge aerosol. The F2299 method requires the use of Optical Particle Counters (OPCs) and permits a wide range of media sample sizes and flow rates. The TSI Model 3160 Automated Filter Tester is an alternative approach permitting the user to conduct monodisperse testing from 15 to 800 nm. The Model 3160 is capable of generating submicron PSL aerosol and is an automated solution.
TSI Solutions for ASTM F2299 Medical Mask Testing
Users have several options when choosing how to conduct F2299-associated medical mask testing. These options may be component-based solutions, or a user may opt for an automated solution.
Component-Based Solutions
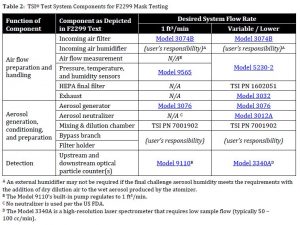
Within the category of “component-based solutions” users are creating test setups that vary slightly from one another. Two examples are shown in Figure 3 and Table 2: a variant with a system flow of one cubic foot per minute, and a variant that permits lower (and/or variable) system flows.
Automated Solution
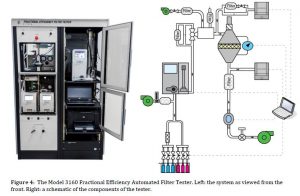
The TSI Model 3160 Fractional Efficiency Automated Filter Tester is a turnkey solution that can provide data very similar to that required by ASTM F2299. The Model 3160 is designed to challenge filter media (and in some cases, finished filters) with monodisperse aerosol in the range of 15 to 800 nm. It generates a true monodisperse aerosol with an electrostatic classifier (EC) and counts particles with dual Condencsation Particle Counters (CPCs). This enables the user to determine the fractional efficiency curve of the filter media under test, which includes determining the most penetrating particle size (MPPS). This is valuable information during the filter media development process, and can also be very useful in quality control efforts. HEPA and ULPA filters can be characterized using the Model 3160, which is capable of measuring 99.9999999%+ of fractional efficiency. The Model 3160 can test filters with up to 100 L/min of flow.
The Model 3160 has the following technical differences with the requirements of ASTM F2299:
- CPCs are used instead of OPCs
- DMA-classified oi and/or salt particles are typically used, but using PSL is possible
- While the 3160 and ASTM F2299 both require a neutralized aerosol, the US FDA requires use of a non-neutralized aerosol
Filtration Efficiency Testing for Surgical Respirators
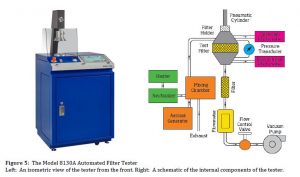
As surgical respirators must meet the same filtration requirements as standard respirators (according to 42 CFR 84 in the US), they are tested the same way. The TSI CertiTest Automated Filter Tester Model 8130A is a widely trusted and used automated filter tester solution for respirator testing, Figure 5 shows a picture and schematic of the Model 8130A.
The Model 8130A generates a polydipserse NaCl or oil challenge aerosol that is detected with two solid-state photometers. Photometers are used for their excellent sensitivity and dynamic range, as well as their ability to measure the large concentrations required for the loading testing of respirators. The 8130A can support flow rates of 10 to 110 L/min and can measure total filtration efficiencies up to 99.9999% during initial penetration and loading tests.
An Anticipated Upcoming Change to ASTM F2100
As of December 2020, ASTM F2100 is being revised with respect to its PFE requirements. To date, ASTM F2100 has relied upon ASTM F2299 as a test method for measuring a medical masks PFE. It is anticipated that likely in the first half of 2021, ASTM F2100 will shift away for requiring ASTM F2299, and will instead require use of a method similar (but not identical to) to that described in 42 CFR part 84. This is being done to more closesly align the filtration test method with the much more common and challenging test required for respirators.
For surgical and procedure masks, this newly-required test method will use the mask’s initial filtration efficiency (when measured with NaCl aerosol, without a loading test). Once this change is made, compliance with ASTM F2100 will call for much the same test equipment than what is currently used for 42 CFR part 84 compliant testing. It is anticipated that the TSI Model 8130A will meet these requirements, as it is already widely used for 42 CFR 84 testing in the United States. Note that individual jurisdictions and regulatory agencies may still impose additional (or different) requirements, so you must verify with the applicable regulatory agencies before proceeding with testing.